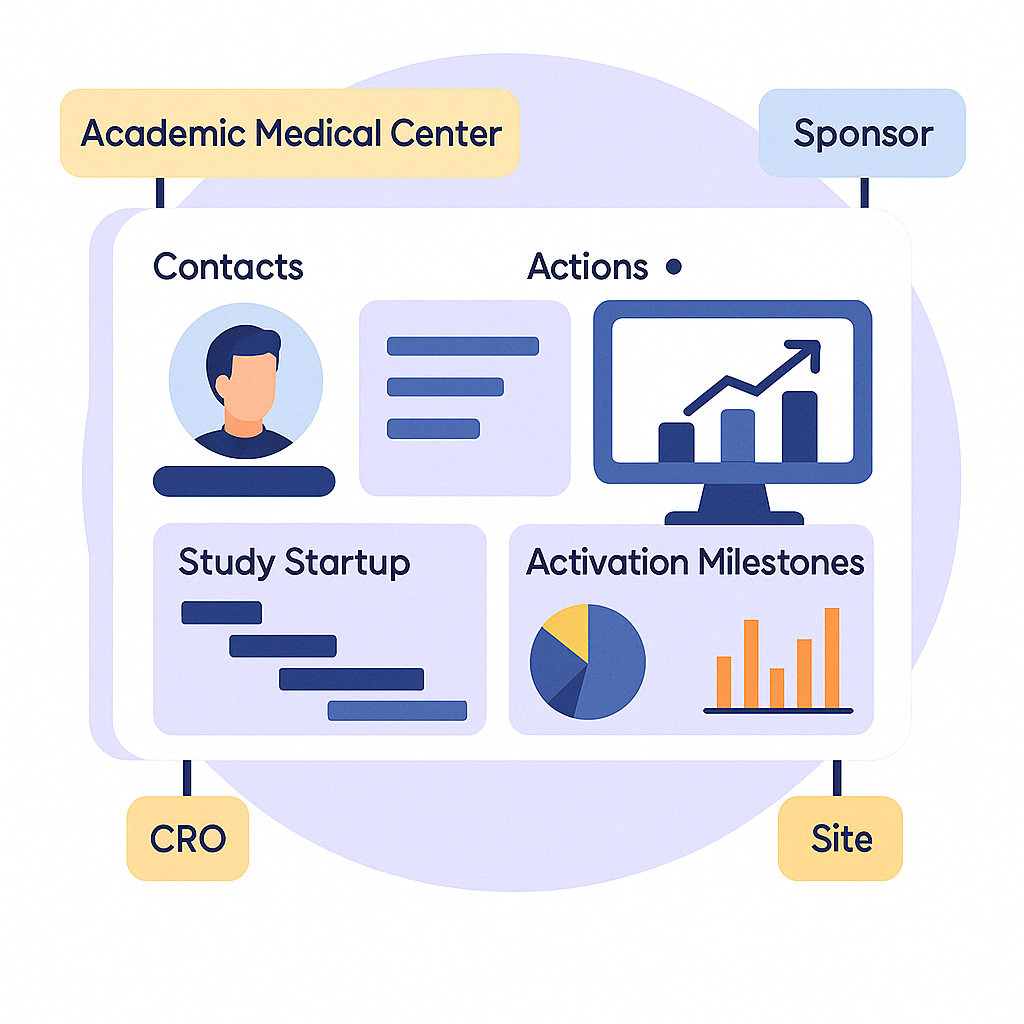
Manage your clinical research pipeline from funding through activation with full transparency at every step.
Bring feasibility, budget, contract, and kickoff tasks into one centralized workspace.
Automate approvals and standardize documentation to stay audit-ready.
Enable seamless coordination between sponsors, sites, and departments with live activity tracking.
Track timelines, identify bottlenecks, and make fast data-driven decisions.
Visualize study startup efficiency, staff workload, and milestone timing to improve outcomes and reporting.

Streamline startup timelines, track site performance, and collaborate directly with research teams—all from one centralized platform.

Coordinate feasibility, budget, and activation across departments with custom workflows and cross-functional visibility.

Gain clarity on startup milestones, reduce communication gaps with sponsors, and stay on track with built-in task management.
By streamlining complex processes, automating workflows, and enhancing collaboration, it empowered research teams to accelerate studies from funding to first patient enrollment, transforming chaos into clarity.
Activation Team