Manual site activation isn’t just tedious—it’s expensive, risky, and invisible. In this post, we expose the hidden costs draining academic medical centers, from the time burned by overworked coordinators to the compliance gaps that threaten audits.

You’ll follow a day in the life of a coordinator, step into the shoes of AMC leadership, and uncover the ripple effects that delays have on patient access and sponsor confidence. Most importantly, we’ll explore how automation can reverse the damage—without disrupting your mission.
The Silent Crisis in AMC Trial Startups
“What if your AMC’s biggest clinical trial bottleneck wasn’t a vendor or a protocol—but a spreadsheet?”
For academic medical centers (AMCs), site activation is the unsung workhorse of trial startup. But when this process is managed manually—through email chains, siloed spreadsheets, and untrackable approval logs—it becomes more than inefficient. It becomes invisible.
And invisible problems are the most dangerous.
For research coordinators, that means hours lost each week to chasing signatures, syncing with multiple departments, or tracking down budget statuses—all while enrollment deadlines loom. For AMC leadership, it means trial timelines drift, sponsor relationships weaken, and compliance risk quietly mounts.
A recent study by Tufts CSDD found that manual startup processes delay up to 70% of clinical trials by 1 to 6 months. These delays aren’t just a headache—they’re a threat to your mission of advancing patient care through research.
This blog breaks down the hidden costs of manual site activation—from lost staff time and budget slippage to patient impact and sponsor attrition. We’ll follow a day in the life of a coordinator, highlight overlooked executive risks, and explore how AMCs can regain control—with tools built for the complexity of academic research.
Inside the Chaos: A Coordinator’s Day
Meet Sarah, a research coordinator at a mid-sized academic medical center. Her calendar is full—but not with patient visits or investigator meetings.
Instead, Sarah spends her day chasing signatures, sifting through email chains over 40 threads deep, and updating a color-coded spreadsheet she built herself to track which sites are IRB-approved, which are stuck in budget negotiations, and which are—somewhere in between.
“It’s like playing a game of broken telephone—with no one keeping score.”
—Anonymous AMC Coordinator
She’s not alone. Industry data shows research coordinators spend up to 20% of their time on manual site activation tasks—the equivalent of one full day per week. At an average salary of $50/hour, that adds up to $15,600 per coordinator per year, per study.
Multiply that by 8–12 trials running in parallel, and the hidden FTE costs become staggering.
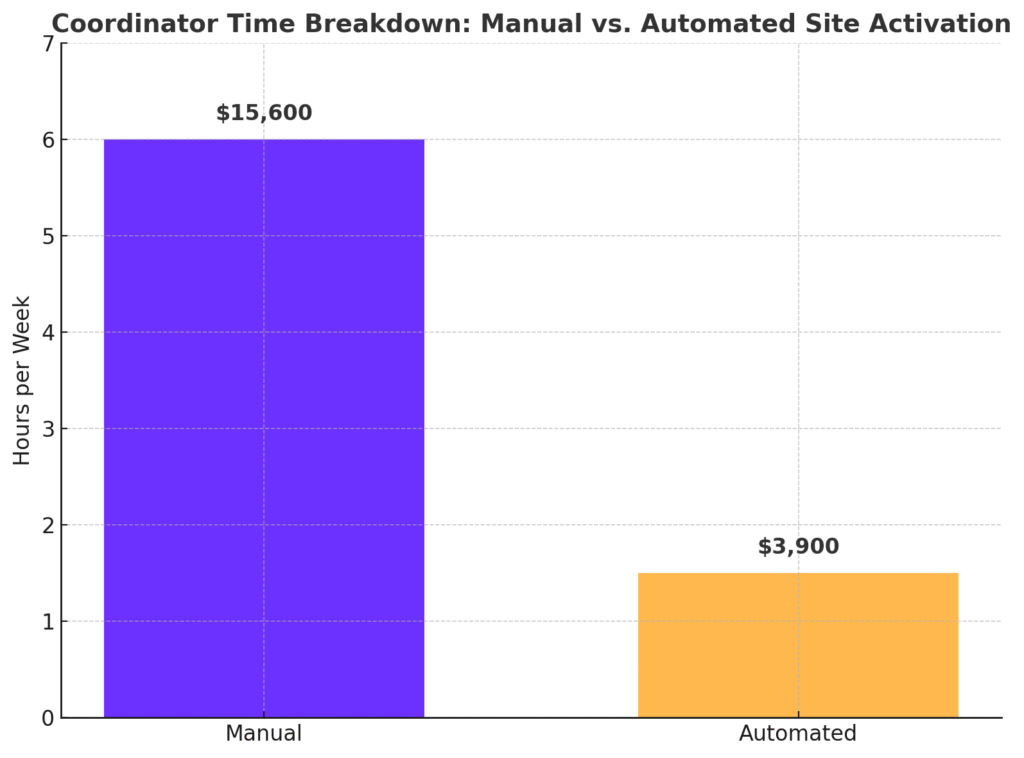
Every 4.5 hours saved per coordinator per week = more patients enrolled, fewer delays
💡 Want to calculate your own team’s hidden cost?
Despite best efforts, Sarah misses a deadline to activate one of the oncology sites. The sponsor flags it. The trial start date is pushed back. Patients wait.
This isn’t inefficiency. This is mission delay.
The Executive View: Reputation, Risk, and Revenue
While coordinators manage the daily chaos, AMC executives face a broader challenge: lack of visibility into where trials stall. With manual workflows, leadership only sees the issues when timelines are already impacted.
Sponsors notice. According to ISR Reports, 40% of sponsors deprioritize AMCs with slow trial startups. And without centralized tracking, manual workflows can result in missed IRB deadlines, outdated documents, and expensive audit surprises.
One AMC incurred $200,000 in penalties after a sponsor audit uncovered undocumented protocol amendments and disorganized budget records.
These aren’t just financial costs. They represent lost trust, damaged sponsor relationships, and internal stress across departments trying to recover.
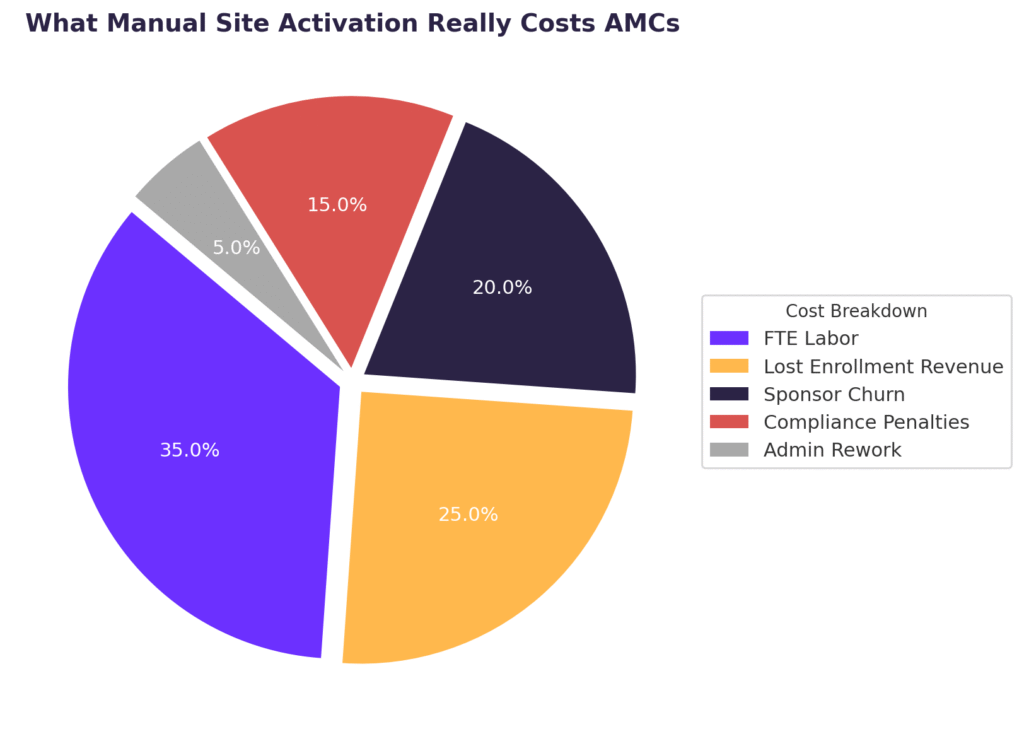
Hidden costs of manual site activation—from labor to penalties and lost sponsor confidence.
📥 Discover Your Site Activation Savings
See how much time and cost your AMC could recover with automation. Download our Infographic on 5 Must Have Features in a Site Activation Platform for AMCs.
Download the InfographicThe Ripple Effect: Patients and Progress
Manual delays don’t just affect internal operations—they can directly impact patients waiting for care and the broader mission of research advancement.
Every week a site activation is delayed, enrollment windows shrink, protocol timelines shift, and eligible patients may miss out on participation.
In one AMC-run oncology trial, a 3-week site activation delay led to a 20% shortfall in target enrollment, meaning fewer patients accessed cutting-edge therapies.
Delayed data collection also slows publications, reduces trial quality, and makes it harder for AMCs to compete with nimble private sites.
This ripple effect—from budget delay to site delay to patient delay—erodes the very outcomes research aims to improve.
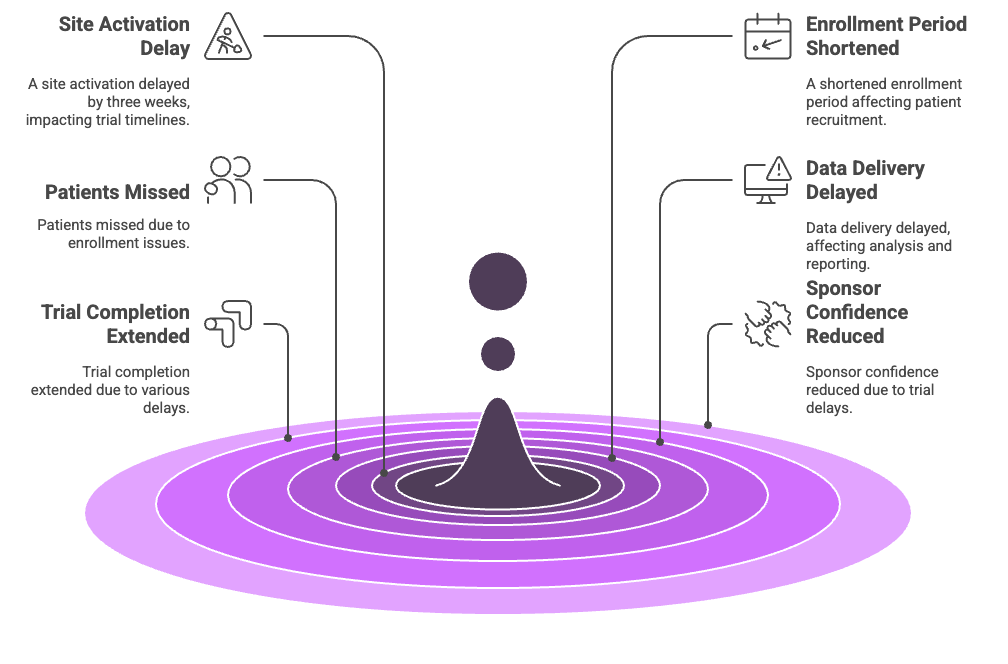
How manual site activation delays ripple across a clinical trial—from protocol to patient to publication
The Turnaround: Automation in Action
After months of missed deadlines and sponsor pressure, Sarah’s AMC adopted a site activation platform designed for academic research workflows.
Within weeks, the change was visible:
- Email chains were replaced with real-time dashboards.
- IRB and budget statuses were visible in one place.
- Approval bottlenecks were flagged before delays occurred.
Result: Site activation time dropped from 12 weeks to 4. Enrollment jumped. Sponsors took notice.
Automation didn’t mean giving up control—it meant gaining visibility, accountability, and time to focus on what matters: patient care and research quality.
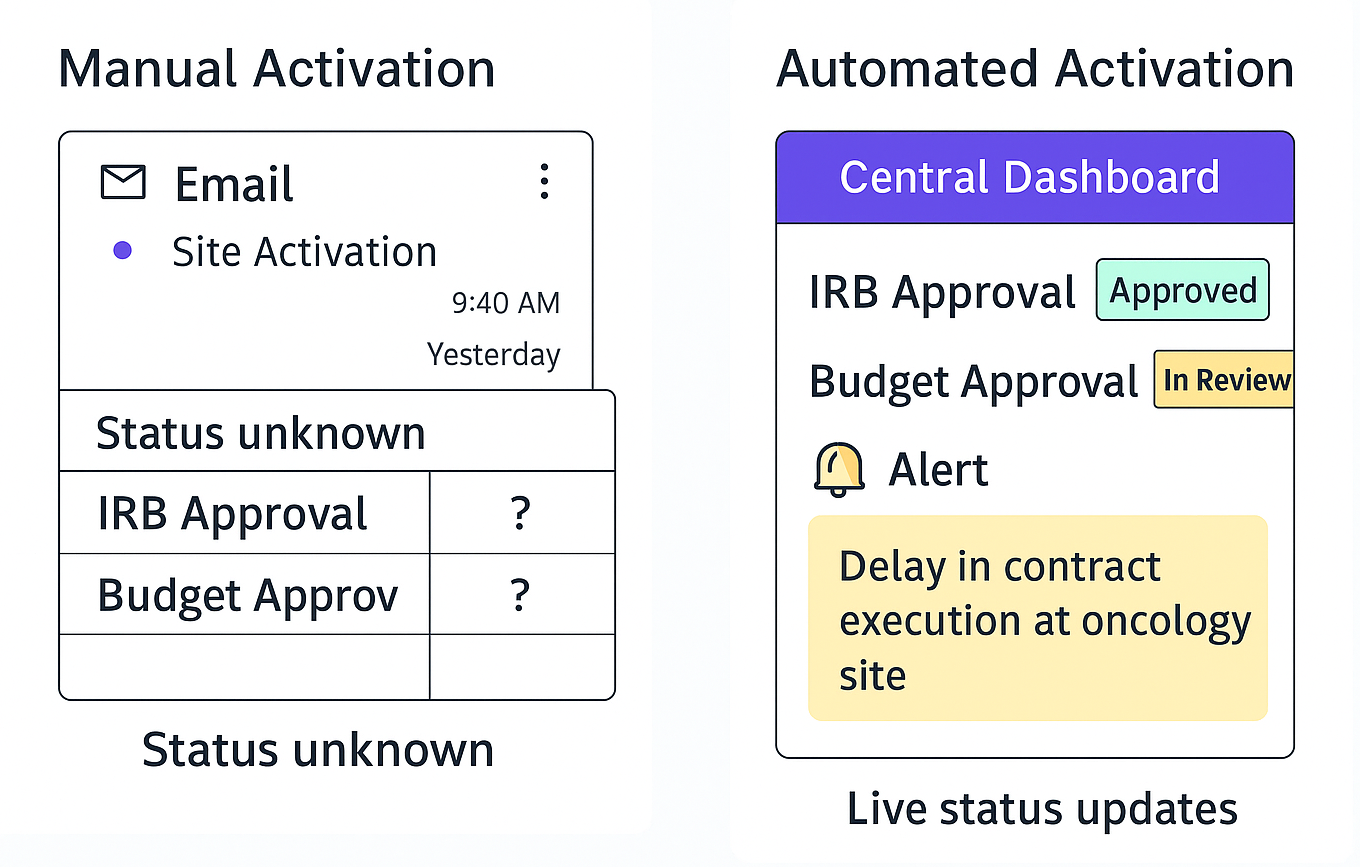
Side-by-side workflow comparison: How automation simplifies activation and accelerates results
Reclaim Your AMC’s Edge
Manual site activation isn’t just a process problem—it’s a risk to your timelines, team morale, sponsor confidence, and, ultimately, your mission.
By making delays visible, automating handoffs, and eliminating guesswork, academic medical centers can compete with speed-driven private sites—without compromising on quality.
“The future of clinical trial startup isn’t faster spreadsheets. It’s smarter systems.”
It’s time to shift from firefighting to forward momentum.
📥 Discover Your Site Activation Savings
See how much time and cost your AMC could recover with automation. Download our Infographic on 5 Must Have Features in a Site Activation Platform for AMCs.
Download the Infographic